How did COVID-19 affect the study?
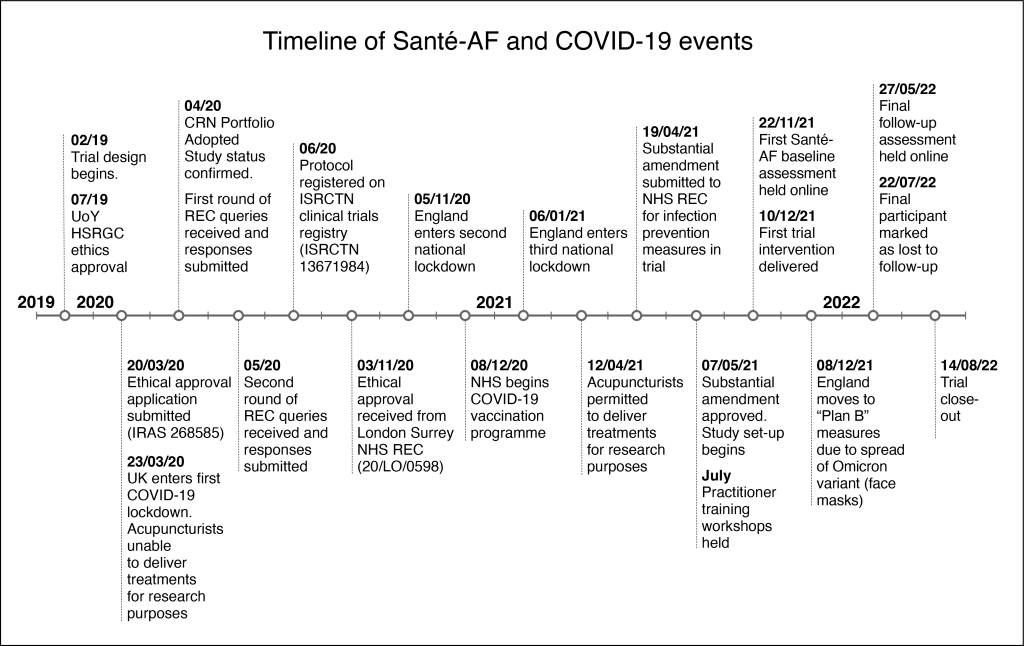
When we began designing Santé-AF, COVID-19 was not even on the horizon. But by March 2020 when the UK went into the first lockdown, it was clear that the study couldn’t go ahead in the timescale planned.
Other work on the project continued, including the systematic review of literature at the start of the study, and the Ethics review by the HRA London (Surrey) Research Ethics Committee. However, the trial itself couldn’t start until lockdown lifted.
As lockdowns didn’t lift sufficiently until the spring of 2021, we used the time to make some changes to the study to maintain participants’ safety, and to also investigate the feasibility of conducting a trial during a pandemic.
How is the risk of COVID-19 transmission minimised in the Santé-AF study?
We made changes to the study to make sure participants’ safety was not compromised:
- We excluded participants who were officially defined as clinically vulnerable, or those who were living with or bubbled with anyone who was clinically vulnerable;
- We moved all the appointments for nutritional therapy from face-to-face to online;
- We required all acupuncturists to follow thorough guidance on cleaning, PPE and other measures to minimise the risk of transmission;
- We moved all study assessments online. For more about the online assessments, please see the study blog on data collection.
Please use the links below to find out more about:
What are acupuncture and nutritional therapy?
